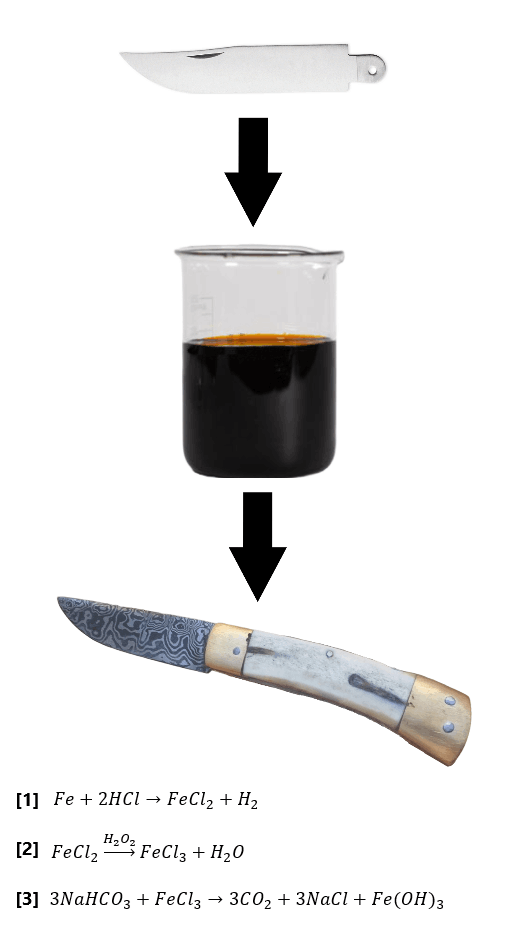
From Steel to Sword!
I am a self-taught blacksmith with a love of blade making!
I designed and fabricated my own forge and power hammer, and I enjoy making blades, jewelry, and sculptures!
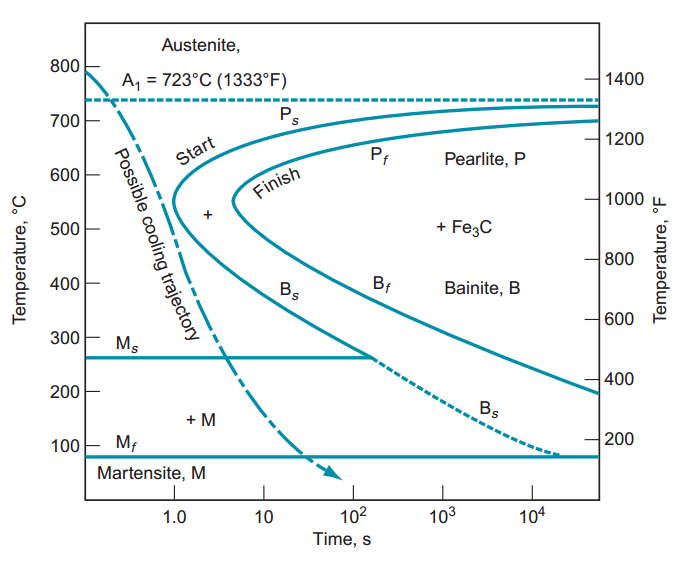
Below is a collection of my work and an overview of the science behind blacksmithing.